Mapping the Pharmaceutical Journey: An AI-Driven PERT Chart for Drug Development
Developing a new pharmaceutical drug is a high-stakes, multi-phase endeavor that demands precision, timeline rigor, and cross-functional coordination. From initial target identification to final regulatory approval, each step must be carefully sequenced and monitored. The complexity of dependencies, regulatory hurdles, and scientific uncertainty makes traditional planning tools inadequate. Enter the Visual Paradigm AI Chatbot — not just a diagram generator, but a collaborative modeling partner that turns high-level concepts into structured, actionable PERT charts through natural conversation.
From Idea to Approval: A Collaborative Design Journey
The journey began with a simple prompt: “Create a PERT chart to map the phases of developing a new pharmaceutical drug from research to regulatory approval.” Within seconds, the AI Chatbot responded with a fully structured PlantUML script, complete with task sequencing, durations, start and finish dates, and responsible stakeholders. This wasn’t just a diagram — it was a living project plan.
But the real value emerged in the conversation that followed. When the user asked, “What factors could delay the regulatory review and approval phase?”, the AI didn’t stop at listing risks. It delivered a categorized, insight-rich breakdown — from data quality issues and safety signals to regulatory backlogs and geopolitical factors — each supported by real-world context and mitigation strategies.
That’s the power of the Visual Paradigm AI Chatbot: it doesn’t just render diagrams. It acts as a modeling consultant, offering expert-level commentary, refining logic, and adapting to follow-up questions like “Explain this branch” or “Refine the dependency chain” with precision and clarity.
Visualizing the Drug Development Lifecycle
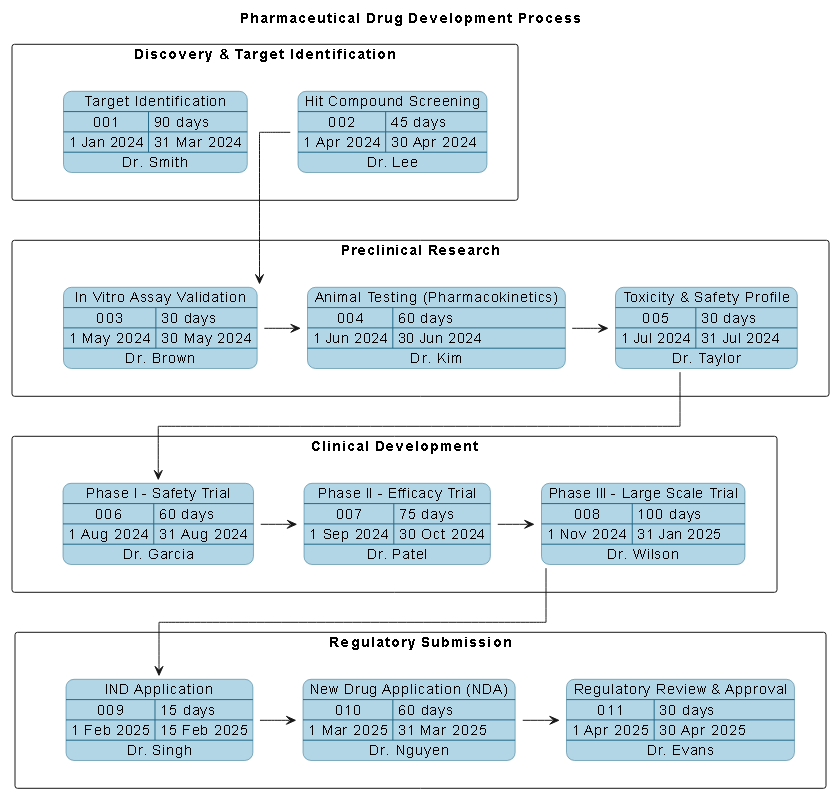
The resulting PERT chart is a comprehensive map of the pharmaceutical development lifecycle, divided into four core lanes:
- Discovery & Target Identification: 90 days from January to March 2024
- Preclinical Research: 120 days of in vitro assays, animal testing, and toxicity studies
- Clinical Development: 235 days across three phases (I, II, III), with escalating sample sizes and complexity
- Regulatory Submission: 105 days from IND application to final approval
Each task is assigned a unique ID, responsible party, and exact timeline. Dependencies are clearly defined — for example, task05 (Toxicity & Safety Profile) must finish before task06 (Phase I - Safety Trial) can begin. This ensures the critical path is visible and the timeline is logically sound.
Decoding the Logic: Why PERT, Not Gantt?
Why PERT over traditional Gantt charts? Because PERT excels at visualizing task dependencies and identifying the critical path — essential when development timelines are fragile and delays compound. In this case, the AI selected PERT because:
- Uncertain durations: Clinical trials and regulatory reviews involve inherent uncertainty, making PERT’s probabilistic approach more realistic than fixed Gantt bars.
- Dependency clarity: The AI explicitly defined each dependency using
$dependency(), ensuring no task starts prematurely. - Stakeholder accountability: Each task includes a responsible lead (e.g., Dr. Smith, Dr. Lee), enabling clear ownership and tracking.
The use of PlantUML syntax with the pert-chart.puml library ensures compatibility, scalability, and consistency across enterprise environments. The AI didn’t just generate a diagram — it built a model grounded in project management best practices.
Conversational Intelligence in Action
What sets Visual Paradigm apart is the depth of insight the AI provides beyond the visual. After the initial PERT chart was generated, the user asked a follow-up question about regulatory delays. The AI responded with a structured, evidence-based analysis — not just a list, but a framework for risk mitigation.
For instance, when discussing safety signals, the AI highlighted how a single adverse event post-trial could trigger a full safety review, potentially delaying approval by months. It then suggested proactive measures like real-time safety monitoring and early engagement with regulatory affairs — insights that would typically require consulting a seasoned project manager.
This isn’t just automation. It’s AI-powered modeling intelligence, where the chatbot acts as a senior project architect, guiding users through complex trade-offs and strategic planning.
More Than a PERT Tool: A Full Modeling Suite
The Visual Paradigm AI Chatbot isn’t limited to PERT charts. It supports a full suite of modeling standards, making it the ultimate platform for enterprise architects and project leaders:
- UML: For software design and system modeling
- ArchiMate: For enterprise architecture and business-IT alignment
- SysML: For systems engineering and complex product development
- C4 Model: For software architecture visualization at multiple levels
- Mind Maps, Org Charts, SWOT, PEST, and more: For strategic planning and ideation
Whether you’re mapping a drug development lifecycle, designing a new software system, or aligning IT strategy with business goals, the AI Chatbot adapts to your language and delivers expert-level models — all through natural conversation.
Conclusion: Build Smarter, Plan Faster, Deliver with Confidence
The pharmaceutical drug development process is one of the most complex and high-risk endeavors in science and business. But with the Visual Paradigm AI Chatbot, teams can transform abstract ideas into precise, actionable models — not through rigid templates, but through intelligent, iterative dialogue.
From the initial prompt to the final regulatory approval phase, the AI didn’t just create a PERT chart. It provided strategic insight, risk awareness, and a living project blueprint — all in real time.
Ready to build your next model with AI intelligence? Explore the full interactive session and see how the AI Chatbot turns your vision into a structured, intelligent model.
